Page 141 - Contributed Paper Session (CPS) - Volume 1
P. 141
CPS1201 M. Iftakhar Alam et al.
whole, the combined criterion with = 0.8 enhances the performance for
Scenario 1, whether D-optimality is penalised or not.
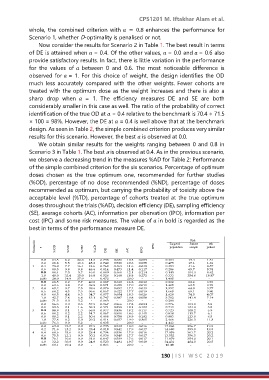
Now consider the results for Scenario 2 in Table 1. The best result in terms
of DE is attained when = 0.4. Of the other values, = 0.0 and = 0.6 also
provide satisfactory results. In fact, there is little variation in the performance
for the values of between 0 and 0.6. The most noticeable difference is
observed for = 1. For this choice of weight, the design identifies the OD
much less accurately compared with the other weights. Fewer cohorts are
treated with the optimum dose as the weight increases and there is also a
sharp drop when = 1. The efficiency measures DE and SE are both
considerably smaller in this case as well. The ratio of the probability of correct
identification of the true OD at = 0.4 relative to the benchmark is 70.4 ÷ 71.5
× 100 = 98%. However, the DE at = 0.4 is well above that at the benchmark
design. As seen in Table 2, the simple combined criterion produces very similar
results for this scenario. However, the best is observed at 0.0.
We obtain similar results for the weights ranging between 0 and 0.8 in
Scenario 3 in Table 1. The best a is observed at 0.4. As in the previous scenario,
we observe a decreasing trend in the measures %AD for Table 2: Performance
of the simple combined criterion for the six scenarios. Percentage of optimum
doses chosen as the true optimum one, recommended for further studies
(%OD), percentage of no dose recommended (%ND), percentage of doses
recommended as optimum, but carrying the probability of toxicity above the
acceptable level (%TD), percentage of cohorts treated at the true optimum
doses throughout the trials (%AD), decision efficiency (DE), sampling efficiency
(SE), average cohorts (AC), information per obervation (IPO), information per
cost (IPC) and some risk measures. The value of in bold is regarded as the
best in terms of the performance measure DE.
Risk
Scenario a %OD %ND %TD %AD DE SE AC IPO IPC Targeted Patient patient
nth
sample
population
0.0 64.3 6.2 20.0 44.6 0.738 0.606 10.3 0.003 - 0.464 13.4 1.31
0.2 68.8 5.5 19.6 45.2 0.749 0.589 10.6 0.005 - 0.475 17.1 1.61
1 0.4 73.0 7.7 16.1 43.6 0.762 0.469 11.6 0.043 - 0.454 41.6 3.57
0.6 80.5 9.8 8.8 46.6 0.814 0.473 11.4 0.117 - 0.286 65.7 5.74
0.8 88.4 7.3 3.7 46.0 0.889 0.460 12.1 0.214 - 0.143 104.4 8.62
1.0 40.0 32.4 15.0 16.8 0.526 0.168 18.8 0.272 - 0.333 354.6 18.87
b(πˆ ) 28.0 14.9 37.9 - 0.472 - 20.0 - - 1.495 - -
0.0 68.5 2.2 7.7 40.0 0.887 0.669 16.8 0.010 - 0.930 62.6 3.73
0.2 69.6 3.8 7.9 39.9 0.871 0.658 17.0 0.011 - 1.405 63.5 3.73
2 0.4 69.2 3.7 7.5 39.6 0.876 0.652 17.2 0.013 - 1.352 64.8 3.77
0.6 68.2 4.5 7.3 36.6 0.867 0.622 17.7 0.019 - - 1.665 69.1 3.90
1.620
4.07
74.5
6.3
34.7
0.604
0.877
0.026
18.3
4.4
66.5
0.8
1.0 42.7 7.4 6.8 13.1 0.747 0.387 19.8 0.058 - 3.782 141.4 7.14
71.5 0.0 5.2 - 0.863 - 20.0 - - 0.288 - -
b(πˆ ) 86.3 0.0 2.3 57.9 0.967 0.866 17.8 0.094 - 0.776 104.3 5.8
0.0
0.2 89.5 0.1 1.6 58.3 0.974 0.858 18.1 0.100 - 0.719 104.8 5.8
3 0.4 88.9 0.1 1.3 56.8 0.977 0.836 18.2 0.117 - 0.723 108.2 5.9
0.6 88.2 0.2 2.2 54.7 0.967 0.800 18.6 0.145 - - 0.810 113.7 6.1
18.9
0.758
0.182
123.0
0.883
0.8
50.6
6.5
0.1
88.2
2.2
0.968
1.0 77.8 0.2 5.0 29.2 0.914 0.657 19.9 0.365 - 2.466 226.3 11.3
76.3 0.0 5.2 - 0.935 - 20.0 - - 1.113 - -
b(πˆ ) 69.0 15.7 0.0 27.9 0.795 0.542 18.0 0.016 - 17.268 356.7 19.8
0.0
0.2 71.6 13.2 0.0 28.4 0.818 0.542 17.9 0.017 - 14.680 355.0 19.8
4 0.4 68.6 15.6 0.0 28.4 0.794 0.536 17.9 0.016 - 17.059 361.7 20.2
0.6 75.5 12.1 0.0 30.2 0.836 0.540 17.7 0.017 - 13.552 356.5 20.2
0.8 76.1 10.6 0.0 31.0 0.847 0.539 17.6 0.017 - 11.879 354.0 20.1
1.0 32.2 30.0 0.0 24.8 0.523 0.491 19.7 0.018 - 34.431 404.0 20.5
b(πˆ ) 60.1 10.4 0.0 - 0.873 - 20.0 - - 10.48 - -
130 | I S I W S C 2 0 1 9